FDA Seriously Considers Allowing Monogamous Married Gay Men to Donate Blood
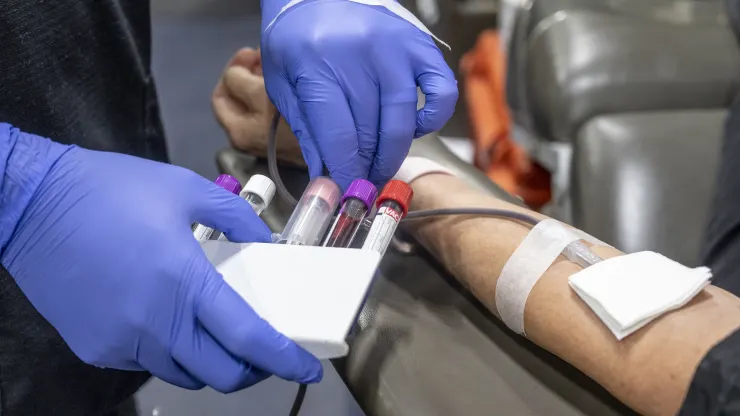 | ||
| A nurse fills test tubes with blood to be tested during an American Red Cross bloodmobile in Fullerton, CA on Thursday, January 20, 2022. |
Paul Bersebach | MediaNews Group | Getty Image
The FDA on Friday proposed new guidelines that would ease restrictions on gay and bisexual men donating blood.
The FDA said the policy would shift to an individual assessment that evaluates risk regardless of gender or sexual orientation.
The American Medical Association and LGBTQ rights organizations have criticized the blood donor restrictions as discriminatory.
A nurse fills test tubes with blood to be tested during an American Red Cross bloodmobile in Fullerton, CA on Thursday, January 20, 2022.
A nurse fills test tubes with blood to be tested during an American Red Cross bloodmobile in Fullerton, CA on Thursday, January 20, 2022.
The Food and Drug Administration on Friday proposed new guidelines that would no longer require gay and bisexual men in monogamous relationships to abstain from sex before donating blood.
The FDA had imposed a lifetime ban on men who have sex with men donating blood during the AIDS crisis in the 1980s. The agency eased the ban in 2015, allowing gay and bisexual men to donate blood if they had not had sex in the previous year.
In response to a blood donor shortage during the Covid pandemic, the FDA further eased restrictions in April 2020 to allow gay and bisexual men who had not had sex in the past three months to donate.
Under the guidelines proposed on Friday, gay and bisexual men who are in monogamous relationships would be allowed to donate blood. But individuals, regardless of gender or sexual orientation, who have recently had anal sex with new or multiple partners would have to wait three months before donating.
CNBC Health & Science
Read CNBC’s latest global health coverage:
CDC urges people with weak immune systems to take extra precautions after Covid subvariants knock out Evusheld
“Maintaining a safe and adequate supply of blood and blood products in the U.S. is paramount for the FDA, and this proposal for an individual risk assessment, regardless of gender or sexual orientation, will enable us to continue using the best science to do so,” said FDA Commissioner Dr. Robert Califf on Friday. The Washington Post reported the news earlier.
The American Medical Association had criticized the FDA’s restrictions on gay men donating as discriminatory.
“At issue is the need to evaluate all potential blood donors on an equal basis based on their individual risk factors and without regard to their sexual orientation or gender identity,” said Dr. Gerald Harmon with the AMA in January of 2022.
The Human Rights Campaign, the nation’s largest organization that advocates for LGBTQ rights, said the FDA proposal is a step in the right direction, but more needs to be done to to remove restrictions.
“We urge the Biden administration to prioritize removing remaining barriers and ask the FDA to move expeditiously while ensuring the safety of the blood supply and a blood donation policy in-line with the science,” said HRC President Kelley Robinson in a statement.
People who are taking oral medications to prevent HIV infection would not be allowed to donate blood for the three months following their most recent dose. Those taking injections to prevent HIV would not be allowed to donate blood for two years following their most recent injection.
These medications, called pre-exposure prophylaxis, or PrEP, can result in false negatives on HIV tests, according to the FDA.
Under the proposed FDA policy, anyone who has tested positive for HIV or taken medicine to treat an HIV infection would be banned from donating blood. People who have engaged in sex work or used illicit intravenous drugs recently would have to wait three months to donate.
Blood banks would still be required to test all donations for HIV as well hepatitis C and B, according to FDA.
Dr. Peter Marks, a senior FDA official, said the agency is evaluating the science to increase the number of people who are eligible to donate blood while maintaining safeguards that ensure the supply is safe for recipients.
“We will continue to follow the best available scientific evidence to maintain an adequate supply of blood and minimize the risk of transmitting infectious diseases and are committed to finalizing this draft guidance as quickly as possible,” Marks said on Friday.
Comments